TMC ACT: Accelerating novel cancer therapies
Let’s say a scientist has invented a drug that shows great promise in the treatment of a certain type of cancer. To bring that novel cancer therapy to market, the scientist must build a company around it and raise enough money to take it from preclinical development to Phase II trials and beyond, when the therapy is tested in humans.
But that’s easier said than done, especially if you’re an academic with no experience starting a company. What’s the best way to get a biotech business off the ground? What are the fundamentals of the drug development process? How do you write a successful grant? Where do you turn for expert advice and mentorship?
A new program, TMC ACT (Accelerator for Cancer Therapeutics), aims to answer these questions and more by providing training, resources and mentoring to Texas inventors and startups looking to bring novel cancer therapies from early drug discovery to commercialization. Funded by a $5 million grant from the Cancer Prevention and Research Institute of Texas (CPRIT), the accelerator is a collaboration between the Texas Medical Center, Gulf Coast Consortia and UTMB Health.

Emily Reiser, Ph.D., senior manager of community engagement at TMC Innovation
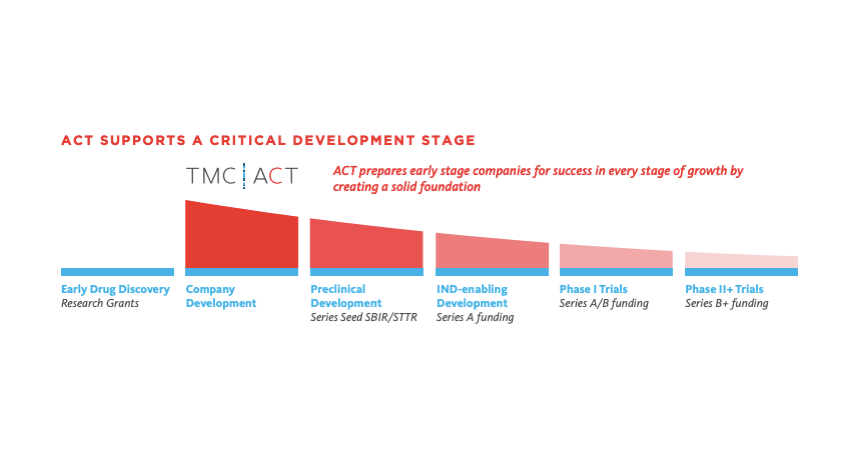
ACT is designed to catch inventors and startups early in the development process, before they make decisions that could cost them unnecessary time and money.
The accelerator “partners with inventors before they’ve built out their teams and mapped out their milestones,” said Emily Reiser, Ph.D., senior manager of community engagement at TMC Innovation. “We will help them write a commercialization grant in the right way and then build and find their team.”
There’s still time to apply to TMC ACT. Applications for the nine-month program, administered by TMC Innovation, will be accepted through September 30. TMC Innovation will manage curriculum development, the mentoring list and an external advisory committee for the program, in addition to hosting workshops.
“The accelerator is going to have the expertise of an incredible mentor-advisor network that’s available because of the TMC,” said Suzanne Tomlinson, Ph.D., MBA, director of research programs at Gulf Coast Consortia for Quantitative Biomedical Sciences. “They already successfully deployed their digital health and device accelerator programs.”
::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
Looking for the latest on the CORONAVIRUS? Read our daily updates HERE.
::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
Leah DiMascio, Ph.D., and Sarah Hein, Ph.D., are the entrepreneurs in residence for ACT at TMC Innovation. They’ll guide the first cohort of the accelerator, which will run from January to September of 2021. Both DiMascio and Hein have founded biotech companies and both understand, firsthand, what inventors need to be successful.

Leah DiMascio, Ph.D., an entrepreneur in residence for the ACT program at TMC Innovation
“When I co-founded my first company, I had already been working in the biotech industry for close to 10 years,” DiMascio said during a recent ACT webinar. “I had developed a pretty good idea of the process to get a drug from the very early stages through an IND and into the clinic. … What I really didn’t understand was the first step of how to start my own company. Who do I talk to? What paperwork do I have to file?”
TMC ACT is designed to take the guesswork out of this sometimes bewildering process.
“Everyone in the program is going to walk away with a strategic plan that’s specifically tailored to their company and their project, and that will include both business aspects and a drug development plan,” DiMascio said. “We will also provide participants with grant writing support to submit CPRIT and SBIR grants, as well as to facilitate connections to VCs [venture capitalists].”
When Hein became involved in her first startup, she discovered it was easy to overlook small steps that could add months to the timeline.

Sarah Hein, Ph.D., an entrepreneur in residence for the ACT program at TMC Innovation
“These are things as simple as creating a positive control for your antidrug antibody formation, or what additional safety assays other than your standard assays might be required for immunotherapies,” Hein said during the webinar. “I had to spend a lot of time sorting out the who: Who do we talk to to get this done? Who are the right vendors? How do I know if they’re reliable?”
In essence, Hein was looking for a protocol that didn’t exist.
With TMC ACT, she said, “We are right now creating that protocol.”
In the world of cancer therapeutics, the financial stakes are high. Series A funding, which usually comes from venture capital firms, is typically $4 million, whereas a digital health company might raise $100,000 to build an app, Reiser said.
“If an investor is putting $4 million into something, they want to know it’s working,” Reiser added. “They need data, but the data has to be repeatable and toxicology- and safety-related.”
The ACT program will help inventors and startups understand what investors need to move forward with a new cancer therapy.
Approximately 20 spots are available in the TMC ACT program. Participants can expect to gain a deep understanding of market research, U.S. Food and Drug Administration (FDA) regulations, intellectual property, licensing, finance and fundraising. They’ll also get an opportunity to build relationships with health care professionals that lead cancer treatment programs at Texas Medical Center member hospitals.
“We’re going to continue to support projects as they turn into companies,” Reiser said. “This unique focus on company development sets the ACT program apart from anything else we are aware of in the world.”




