Fighting Cancer From Within

For decades, the standard of care in cancer treatment consisted of three major pillars: surgery, radiation and chemotherapy. In recent years, however, another type of treatment—immunotherapy—has grown in leaps and bounds, offering new hope to cancer patients around the world.
Over the past year alone, the FDA approved two immunotherapy drugs for use in melanoma treatment, and in March, one of the drugs was also approved for lung cancer treatment. The field of immunotherapy is not new, but this swift progress comes after years of disappointment and unmet potential. Just as hope for the future of immunotherapy began to fade, groundbreaking discoveries made by James P. Allison, Ph.D., chair of the immunology department at The University of Texas MD Anderson Cancer Center, transformed it into one of the most promising frontiers of cancer treatment.
The idea of using a patient’s own immune system to fight cancer dates back to the late 1800s. Like so many great medical advancements before and after, the discovery of immunotherapy happened largely due to chance. William B. Coley, M.D., a bone surgeon at Memorial Hospital in New York City, noticed that cancer patients who developed bacterial infections after undergoing surgery fared better than other patients. He hypothesized the infection awakened the immune system, which then fought off cancer cells while also fighting the infection.
Coley tested his theory by injecting bacteria in patients with inoperable cancerous tumors. He achieved successes with the method—notably, one young patient with a malignant tumor survived another 26 years after Coley injected him with “Coley toxins.”
Coley’s work created the foundation for the immunotherapy of today, but years passed before significant progress was made in the field. By the early 1900s, radiation therapy entered the scene and quickly became the go-to treatment, alongside surgery. In the 1960s, chemotherapy became the third pillar of treatment.
“These are the three modalities that have persisted,” said Allison. “But of course, they have things wrong with them.”
Surgery, Allison explained, requires early detection—if you can’t get all the tumors before the cancer spreads, surgery is not curative. Radiation treatment poses the same problem. Chemotherapy is successful in curing certain types of cancers, but brings with it terrible
side effects.
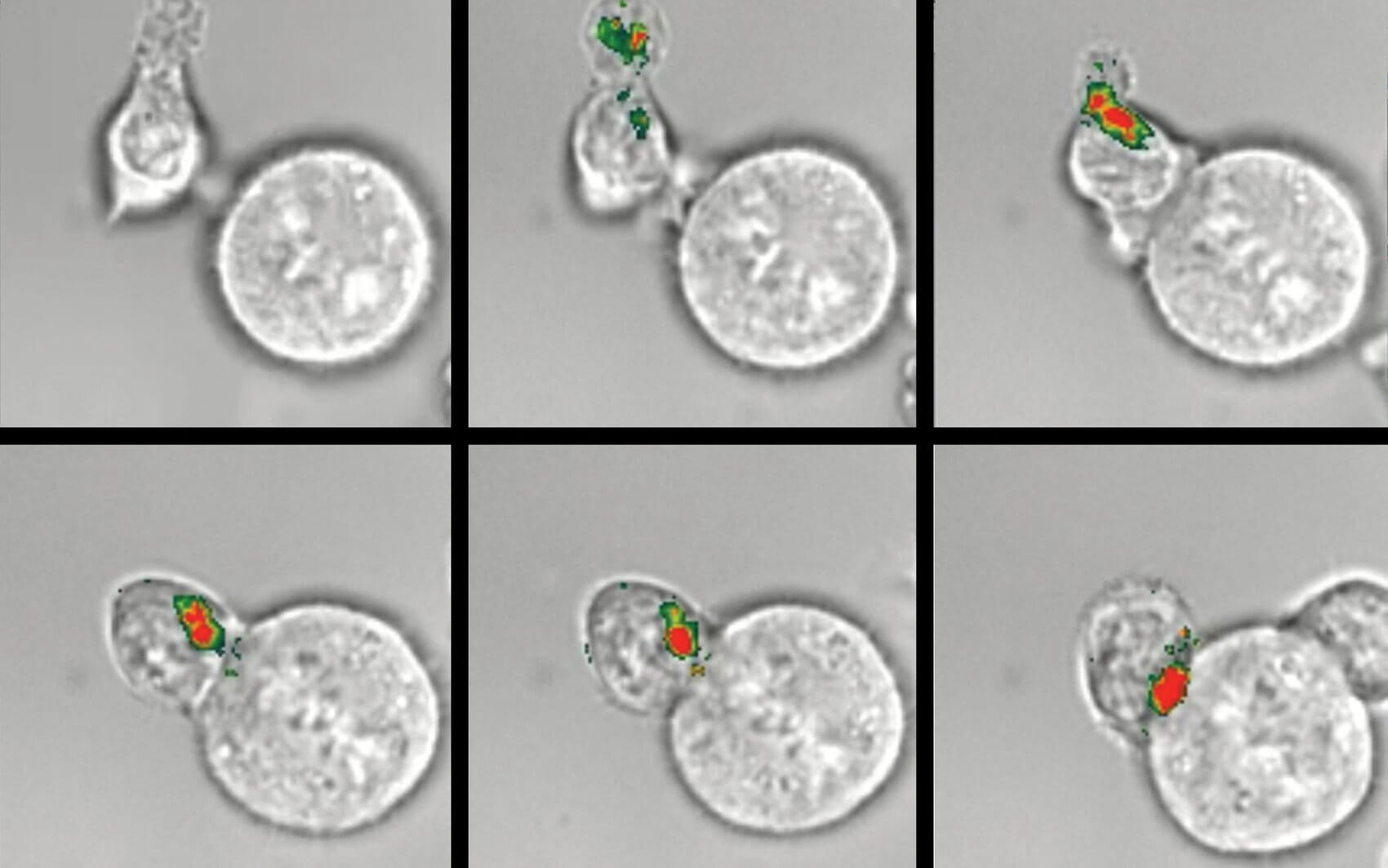
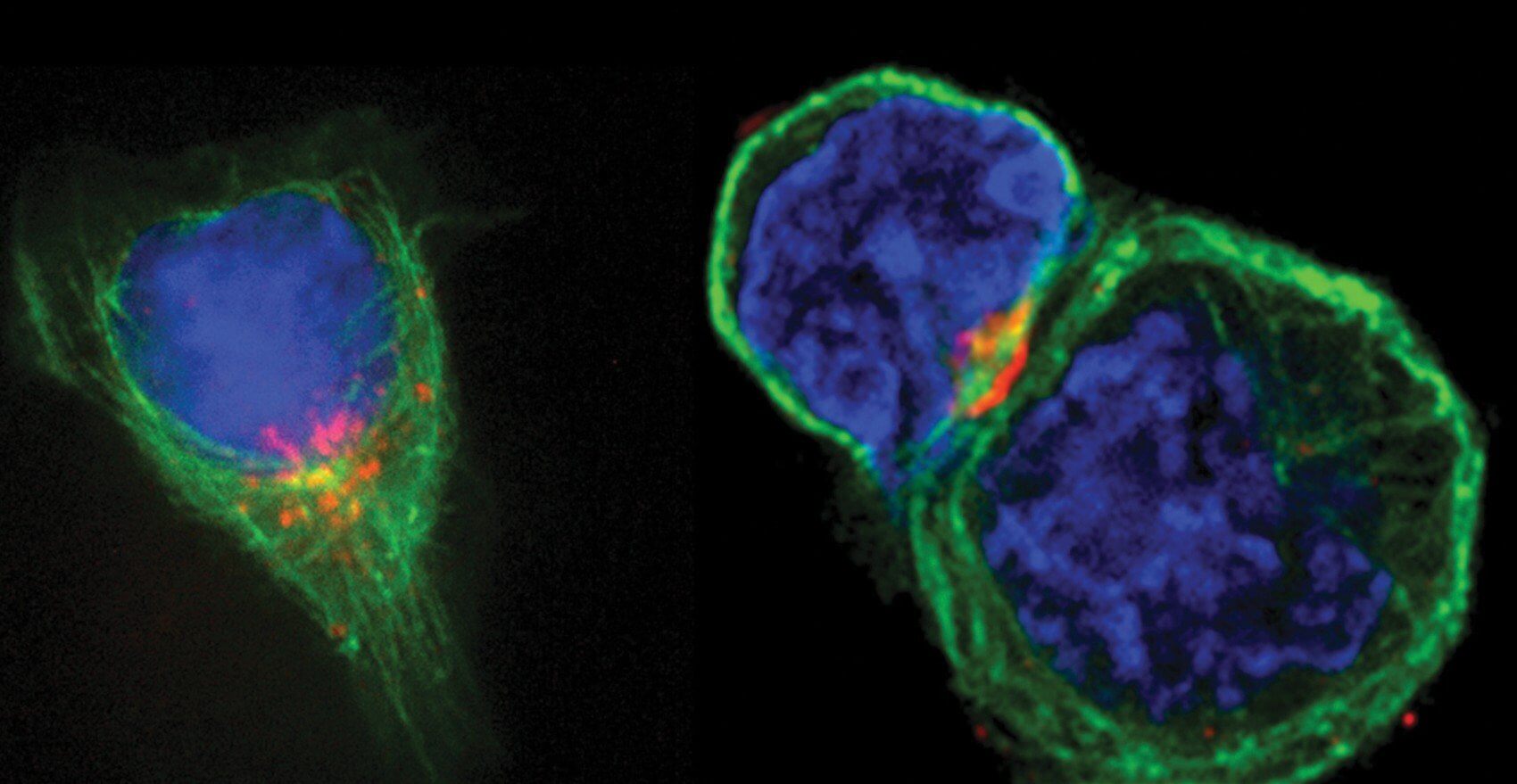
Still, the triumphs of these forms of treatment caused immunotherapy to fall by the wayside, in part because there seemed to be a missing piece in the puzzle of how to achieve a durable immune response. That changed in the 1990s, when Allison and his team found that a molecule on T-cells, the immune system’s attack cells, acts as a brake on the immune response.
Before Allison’s discovery, “we didn’t understand the basic biology of how T-cells were regulated,” said Padmanee Sharma, M.D., Ph.D., professor of genitourinary medical oncology and immunology at MD Anderson. “We tried to give vaccines to turn on the T-cells and attack the tumor, but they didn’t work.”
For years, clinical trials focused on engaging T-cell receptors failed. The immune system would respond for a time, but would eventually shut off. No one understood why, until Allison and other researchers identified the first set of brakes on T-cells, CTLA-4, now known as a “checkpoint molecule.”
“CTLA-4 interferes with the gas pedal of the T-cells and takes the gas off after a while,” said Allison. His next insight was critical: could turning off those brakes on the immune system be a way to attack cancer? Allison and his team shifted focus to searching for ways to block CTLA-4, thus removing the brakes and allowing T-cells to respond freely. This type of treatment became known as immune checkpoint blockade.
“That was the paradigm shift,” said Sharma. “From trying to turn on T-cells, we’re now trying to block inhibitory pathways. People kept saying, ‘We need to harness the immune response.’ Actually, we’re not trying to harness or turn it on, we’re just trying to unleash it. It’s ready to go, it just has a lot of brakes around it.”
Allison developed an antibody to block CTLA-4, which turned into the drug ipilimumab, used to fight metastatic melanoma. Ipilimumab, now known as Yervoy, showed unprecedented results.
“In a study tracking 5,000 patients who received the drug,” said Allison, “22 percent are alive 10 years after they stopped therapy.” At the time the drug was developed, the median survival rate for late-stage melanoma was 11 months.
In 2014, the FDA approved the drugs Keytruda and Opdivo, which inhibit another checkpoint molecule, PD-1, for the treatment of metastatic melanoma. In March 2015, Opdivo was also approved for lung cancer treatment. When taken together, anti-CTLA-4 and anti-PD-1 drugs have been shown to dramatically increase survival rates.
“The combination of the anti-CTLA-4 and the anti-PD-1 in melanoma gives you 50 percent of patients responding,” said Allison. “The whole goal now is to extend this to more types of cancer and find the right combinations where we can get the survival rate even higher.“
One key step MD Anderson took to enhance immunotherapy research for treating a variety of different cancer types was establishing it as a platform in the institution’s Moon Shots Program, launched in 2012.
“The Moon Shots are designed to decrease cancer mortality by having major ideas implemented, either through prevention, early detection, or treatment,” said MD Anderson President Ronald A. DePinho, M.D. “We call it research-driven patient care. We’re not just focused on standard of care. We’re not just focused on experimental modalities. We really bring the whole package together for patients.”
Allison is executive director and Sharma scientific director of the immunotherapy platform of the Moon Shots Program.
“The Moon Shots programs are focused on particular tumor types—for example, women’s cancer or prostate cancer—and immunotherapy plays a role in all of those,” said Sharma. “Trying to integrate an immunotherapy treatment strategy for each tumor type is very important in helping the Moon Shots goals be accomplished, which is, of course, to eliminate and reduce mortality and morbidity of cancer.”
To that end, MD Anderson is engaged in 50 or more clinical trials related to immunotherapy at any given time, in addition to the hundreds of clinical trials MD Anderson manages overall. In fact, the institution’s cancer clinical trial program is the largest in the country.
The opportunity to conduct clinical trials and the symbiotic relationship between clinicians and researchers were some of the biggest selling points when Allison decided to move to MD Anderson. Allison’s initial cancer research discoveries took place while he was working in the Cancer Research Laboratory at the University of California, Berkeley. He later became chair of immunology at the Memorial Sloan-Kettering Cancer Center before arriving at MD Anderson in 2012.
“I came here specifically to do this work,” said Allison. “At Berkeley, it was not possible to do any human work.”
Between the clinical work, the surgical work to obtain tissue and tumor samples, and the research done in Allison’s and Sharma’s labs, teamwork plays a vital role in taking on the breadth of trials MD Anderson conducts.
“We try to identify clinicians who are really interested—an oncologist using drugs to treat patients, who’s interested in immunotherapy— and also try to find surgeons who will work with us, a pathologist who will help us get the tissues. Then we need to have research nurses,” said Allison. “It’s very complex. This isn’t being done anywhere else on the planet, as far as I know. At least not at this scale.”
Allison’s and Sharma’s labs conduct different, but related, research. Sharma works closely with patients. She sees patients one day a week, predominantly bladder and kidney cancer patients. All patients are evaluated for possible inclusion in clinical trials, and many are enthusiastic about the opportunity to help further research at MD Anderson.
“They want us to learn so we can do better for future generations,” said Sharma. “Especially with immunotherapy, because a lot of patients have heard about the potential benefits.”
Sharma’s lab does translational work, trying to understand what is happening in the patients—what the immune response is when you give a certain drug or drug combination, or how the diseases progress.
“That’s what I call ‘hypothesis-generating information,’” she said. “I can take all the data I learn from patients and say, ‘This information correlates with when the patient did well.’ But it doesn’t really link A to B in terms of why the patient did well. You really need mouse models to understand that.”
That’s where Allison’s labs come in to play.
“They can help us test that hypothesis,” said Sharma. “They’ll use the right mouse model and say, ‘Yup, if you don’t have this gene you don’t get a response.’”
Allison runs two labs, one working in conjunction with Sharma, and his own personal research lab, where he and his team search for new checkpoint targets and try to determine combinations of treatments to further improve survival rates.
The search for effective combinations not only includes pairing multiple immunotherapy treatments, like the anti-CTLA-4 and anti-PD-1 drugs, but also combining immunotherapy with other types of treatment. Allison and Sharma recently published papers in the journals Cell and Science to discuss the potential of combining genomically targeted therapy with immunotherapy.
“Don’t even try to make those targeted genomic therapies curative, because they’re not,” said Allison. “There are too many resistance factors. Let’s start making rational combinations.”
For several years, genomically targeted therapies have been touted as the next big thing in cancer treatment, because they garner remarkable responses in a majority of patients. The downside? Those responses don’t last.
“The genome of cancer cells is really unstable,” said Allison. “By the time you find one you can attack, there are probably other mutations that will take over. You’re always following behind.”
If combined with immunotherapy, however, Sharma and Allison suggest it would be possible to get a durable response from a high percentage of patients.
“Because immunotherapy works not by targeting the cancer cell but by targeting the immune system, we thought the combination of these two things would be very powerful,” said Sharma.
A patient could begin with a genomically targeted therapy to kill tumor cells, which would release antigens for the T-cells to attack and associate with tumors.
“While the tumor cells are dying, you can bring in immunotherapy and start the whole immune process,” said Sharma. “You would have a durable response, because immunotherapy allows for the development of memory cells.”
Sharma used the polio vaccine as an example. If you received the polio vaccine as a child, you will still have an immune response as an adult. If you encounter polio, your immune system would remember it and kick in to get rid of it, preventing you from contracting the disease.
“If we can get that to happen with cancer and form memory T-cells, you would have that durable response and you wouldn’t have patients relapsing with their disease,” said Sharma.
In fact, memory is one of the biggest perks of immunotherapy.
“Once you get a big T-cell response, a certain population of those cells acquire stem cell-like capabilities,” said Allison. “They’re with you for the rest of your life. If the tumor comes back, they get turned on again and kill it.”
Despite its recent progress, the field of immunotherapy still has obstacles to overcome in order to reach its full potential. Federal funding is currently “overwhelmingly directed toward genomically targeted therapies as compared to immune checkpoint therapies,” as Allison and Sharma wrote in their Cell article. They suggest more funds be allocated toward immunotherapy research, given its potential and recent successes.
One of the reasons for this imbalance in funding is the fact that immunotherapy experienced years of failures before Allison’s discoveries helped revitalize the field.
“Immunotherapy lost credibility as being something that was really going to make a difference in cancer,” said Sharma. “The genomically targeted therapies came along more recently, but showed benefit so quickly. Immunotherapy couldn’t say that. But this is a different kind of immunotherapy.”
Though it may take time to convince everyone of the new potential in these immune checkpoint therapies, the message appears to be spreading. Allison and Sharma both noted one significant measurement of how far immunotherapy has come over the last decade is evident at the conferences they both attend, such as recent annual meetings of the American Society of Clinical Oncology.
“When I started going to these meetings in 2004, 2005, the immunology sessions would be in a small room. You’d be lucky if there were 50 people there,” said Allison. “Two years ago, when the combination of ipilimumab and anti-PD1 was presented, it was in this huge theater where there were over 3,000 people.”
The most important change in this new era of immunotherapy research, however, can be seen in the clinic and the lab. Even as recently as five years ago, finding clinicians to provide tissue for research was more difficult. These days, many are eager to participate.
“Right now there is so much enthusiasm,” said Allison. “That’s one thing I’ve sensed since coming here. Everybody wants to help—they all see what it’s doing for their patients.”