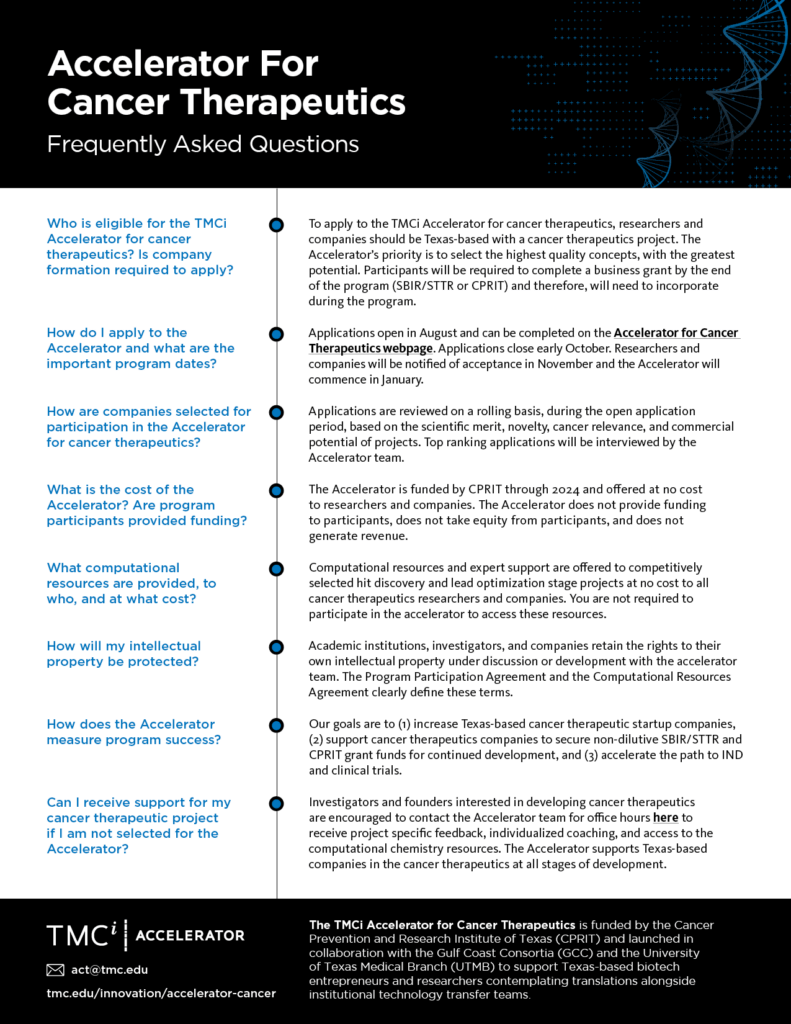
Accelerator for cancer therapeutics
Investigators and early-stage biotechnology company executives can access guidance on clinical and business development through the Accelerator.
The TMCi Accelerator for Cancer Therapeutics is funded by the Cancer Prevention and Research Institute of Texas (CPRIT) and launched in collaboration with the Gulf Coast Consortia (GCC) and the University of Texas Medical Branch (UTMB) to support Texas-based biotech entrepreneurs and researchers contemplating translations alongside institutional technology transfer teams.
“The TMCi Accelerator for cancer therapeutics program has been a transformative experience for me. In a span of 5 months, I have co-founded a company with one of my advisors in the Accelerator; have been taught by experts in the field on topics as varied as venture capital funding, intellectual property, manufacturing, regulatory, and clinical trial design; and have had tremendous mentoring throughout the entire process. I highly recommend the Accelerator for cancer therapeutics for any cancer researcher with aspirations of translating their scientific discoveries.”
Andrew Koh, MD
Associate Professor, Pediatrics and Microbiology
University of Texas Southwestern Medical Center
Curriculum
During the nine-month Accelerator, participants will gain a deep understanding of market research, FDA regulations, intellectual property, licensing, finance, fundraising, legal, and other critical areas that will propel your business for long-term success. Program participants will develop and incorporate an integrated strategic plan that guides their company’s business and drug development efforts. The Accelerator culminates with at least one grant submission, and an option to pitch to investors, corporate partners, media, and other influential guests.
Topics include:
- Target Product Profile
- Intellectual Property
- Designing Efficacy Studies
- Lead Optimization
- Venture Investment
- Term Sheets
- Non Dilutive Funding
- Strategic Partnerships
- Milestone Development
- Financial Planning
- Clinical Trial Design
- Manufacturing
Network
The TMCi Accelerator for cancer therapeutics External Advisory Committee is comprised of experts in cancer therapeutics commercialization who are committed to ensuring that program participants have the highest quality experience and resources to advance cancer drug commercialization efforts within Texas.

Casey Cunningham, MD

John Flavin

Dan Hargrove, JD, LLM

Ann Tanabe

Dan Watkins, PhD
Cohorts
Investigators and early-stage companies participating in the TMCi Accelerator for cancer therapeutics cohorts represent a wide array of focus areas, including immunotherapy, cell therapy, targeted therapy, diagnostics, RNA modification, cancer pain, delivery platform, and drug platforms.
Key Program Benefits
The Accelerator is dedicated to cancer therapeutic development and was designed and modeled based on learnings from our Accelerator for HealthTech. Our flexible model and no equity to participate welcome projects from academic investigators to early-stage biotech companies. Participants can expect:
- Curated mentor network and dedicated entrepreneurs-in-residence to enable progress toward a comprehensive product and business development roadmap
- Identification of and plan to address critical gaps and key experiments to enable funding
- Proximity to world-class researchers and experts in the Texas Medical Center
- GCC Drug Development Core Network and GCC training and resources
- JLABS@TMC Lab space
- Dedicated computational chemistry resources
- Grant writing support to seek non-dilutive funding
- Access to competitive intelligence
- Venture capital relationship development and pitch opportunities
- Program participant portal
Program Information
The Accelerator team supports investigators, institutional technology transfer teams, and companies working on cancer therapeutics. Sign up below for office hours and learn more about the Accelerator and computational chemistry resources.

Program portal
Current Accelerator participants, click the button below for access to resources:
In collaboration with